Keywords: medical devices regulation, MDR, post-approval studies, PMCF, PCMF Investigation, clinical investigation, GDPR
Researchers beware! The new European Union Medical Devices Regulation1 comes into force on the 26th May 2021 and it brings significant changes for post-approval studies.
According to the European Commission2, the main reasons why we needed this new regulation were due to “problems with divergent interpretations of the current Directives3, 4, as well as incidents concerning product performance, which highlighted certain weaknesses in the legal system. Revision of the legislation was necessary to reinforce high standards of product quality and safety and consolidate the role of the EU as a global leader in the medical devices sector.”
My (often too optimistic) expectation for EU Regulations is that they will herald a new era of harmonisation, consistency, conformity and simplicity. That was my hope for the data protection requirements in post-approval studies when the GDPR5 came into force in May 2018. Such was the naivety of my optimism. Whilst the GDPR did remove the need for notifications to/ approvals from national data protection agencies, it also made allowances for Member States to create their own requirements6…cue weary sigh.
Fast forward to May 2021. The medical Devices Regulation promises to harmonise the requirements for clinical trials of medical devices (clinical investigations) and provides clear guidance within the text of the Regulation and in associated guidance documents7 (see figure 1).
All good so far.
But what if you’re interested in conducting studies of CE marked medical devices used within their intended purpose, so called post-market clinical follow-up investigations (PCMF Investigations)? Well…that’s where things get interesting (see figure 1).
The Regulation imposes a harmonised 30 day notification system for PCMF Investigations (post-approval studies) that “involve submitting subjects to procedures additional to those performed under the normal conditions of use of the device and those additional procedures are invasive or burdensome”2.
Again, all good so far.
So…what are the requirements for PCMF Investigations that don’t involve additional procedures or burdens? They aren’t covered by the Regulation. You’ll need to check the local regulatory requirements in each country. This certainly removes the issue of “divergent interpretation”, but also leaves the study sponsor with the headache (pain, frustration, tears, angst) of checking the regulatory requirements of every country…cue weary sigh.
To be fair to the European Commission, the new EU Medical Devices Regulation does solve the “problems with divergent interpretations of the current Directives” with respect to clinical trials of medical devices (sort of – see figure 1; clinical investigations). But it would also be fair to say that this is not the case for post-approval studies (PCMF investigations). My fear is that we’ve created a post-approval ‘monster’ that will result in significantly different requirements for non-interventional studies of medical devices versus drugs.
It was already complicated enough…mind blown! So, here’s a little flow chart (who doesn’t like a flow chart?) to help get your mind around this brave new medical device world
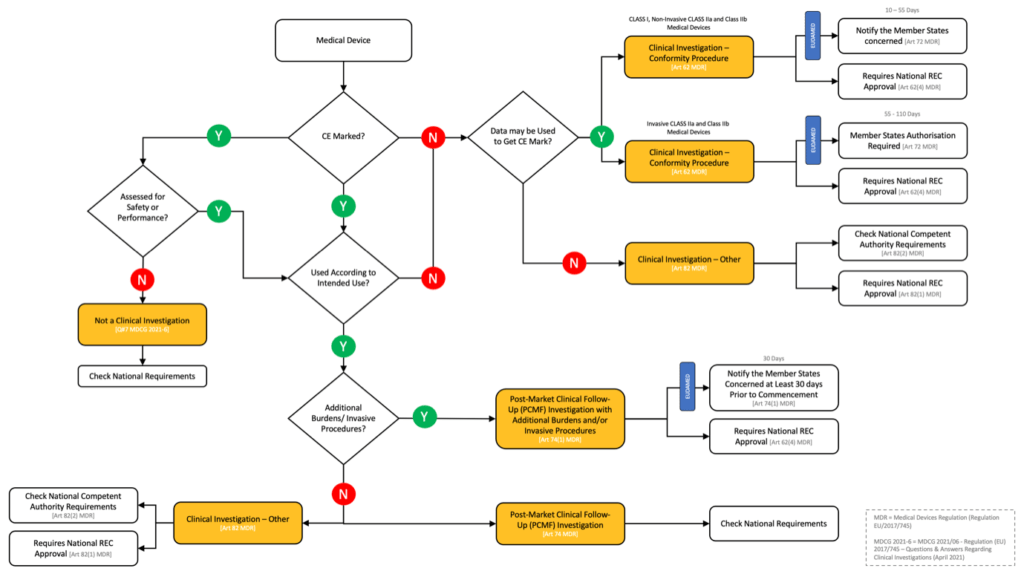
Figure 1 – EU Medical Devices Regulation: Summary of the Clinical Investigation Classification and Submission Pathways
[Click here to download a high definition copy of Figure 1]
References:
1. European Union Medical Devices Regulation (Regulation EU/2017/745): https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0745-20200424
2. European Commission – Medical Devices – New Regulations: https://ec.europa.eu/health/md_sector/new_regulations_en
3. Medical Devices Directive (MDD) (Directive 93/42/EEC): https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:01993L0042-20071011&locale=en
4. Active Implantable Medical Devices Directive (AIMDD) (Directive 90/385/EEC): https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:01990L0385-20071011&locale=en
5. General Data Protection Regulation (GDPR) (Regulation 2016/679/EU): https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02016R0679-20160504&qid=1532348683434
6. Article 89 of the GDPR: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02016R0679-20160504&qid=1532348683434
7. European Commission – Medical Devices – Guidance – MDCG Endorsed Documents and Other Guidance: https://ec.europa.eu/health/md_sector/new_regulations/guidance_en
